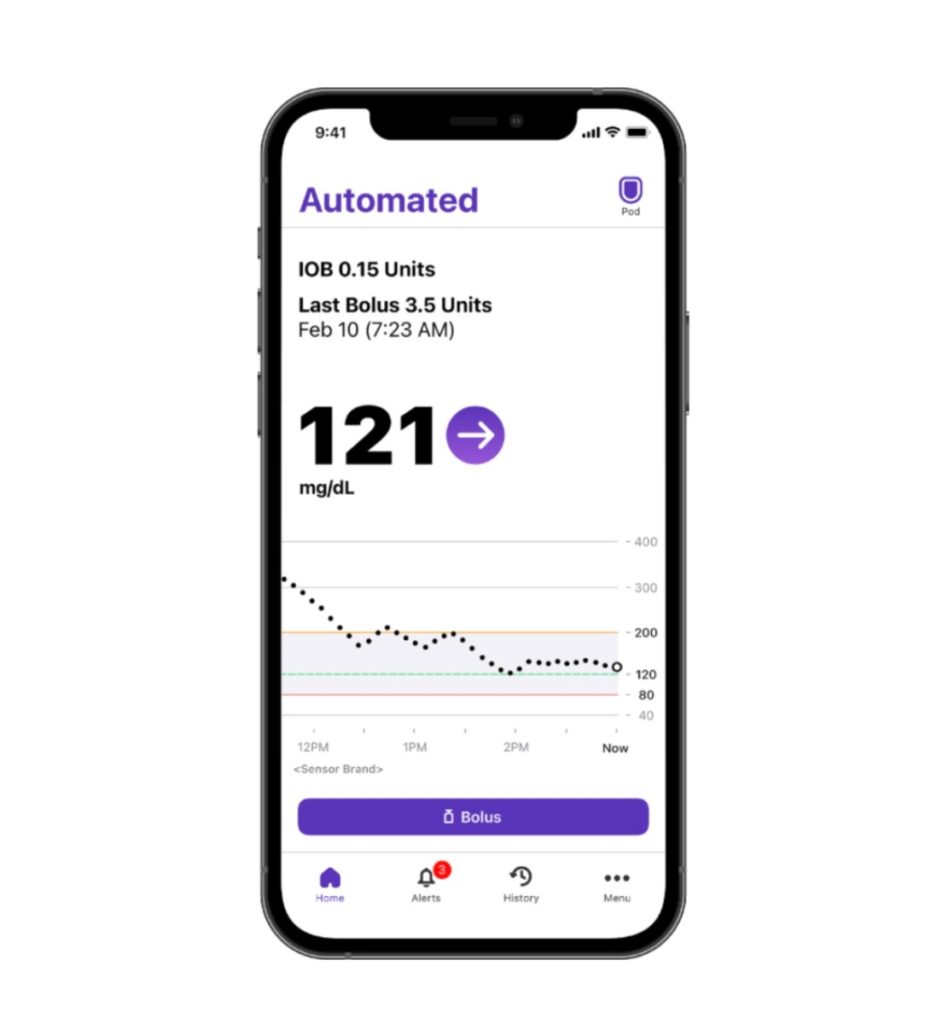
Insulet, which specializes in tubeless insulin pump technology with its Omnipod brand of products, today announced it has received FDA 510(k) clearance for the Omnipod 5 App for iPhone.
This makes Insulet the first and only company to offer a tubeless automated insulin delivery (AID) system with full control from a compatible Android and iOS smartphone, according to Eric Benjamin, executive vice president, chief product & customer experience officer.
Since its full commercial launch in August 2022 in the United States, the Omnipod 5 Automated Insulin Delivery System (Omnipod 5) has included the option for smartphone control for customers using compatible Android devices. With today’s announcement, the Omnipod 5 Pod will soon be controllable from a compatible iPhone, providing even more options for users who prefer not to carry an extra device.
Benjamin says the Omnipod 5 App for iPhone offers the same functionality as the Omnipod 5 App for Android, plus additional capabilities. Users will have access to a new custom foods feature with the ability to save carbohydrate data for favorite foods, snacks, or meals that are consumed frequently.
The Omnipod 5 App for iPhone will launch first with the Dexcom G6 Continuous Glucose Monitoring System integration, with a full market release in 2024. Users of the Omnipod 5 System can download the iPhone App free of charge once available.
People with type 1 diabetes who are interested in Omnipod 5 should contact their healthcare providers to learn how to get started on the system today. The Omnipod 5 App for iPhone will be a simple transition for those with compatible devices once available.
Insulet will inform the diabetes community of the release timing closer to the date. To stay informed of Insulet’s progress with future innovation, visit Omnipod.com/innovation.
Article provided with permission from AppleWorld.Today